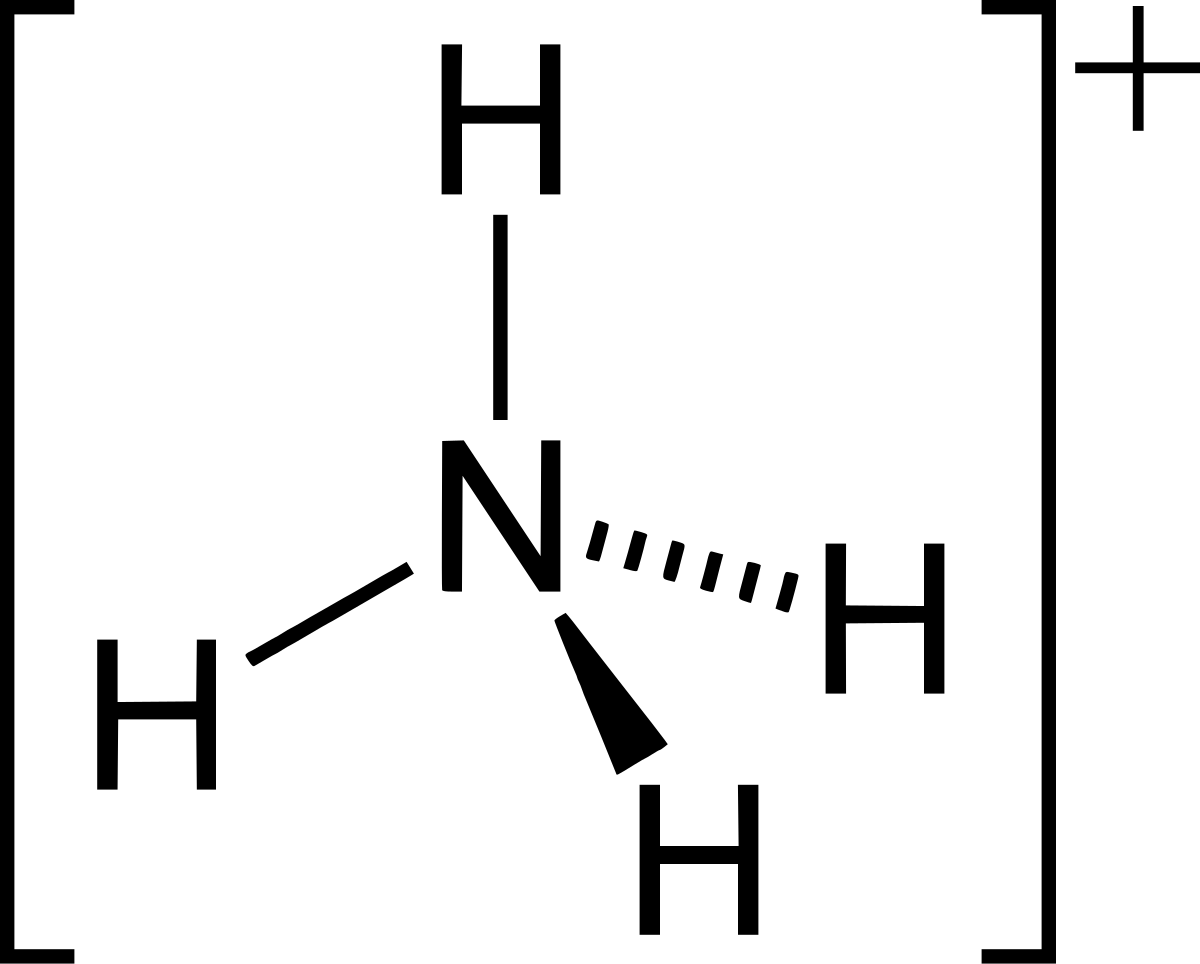
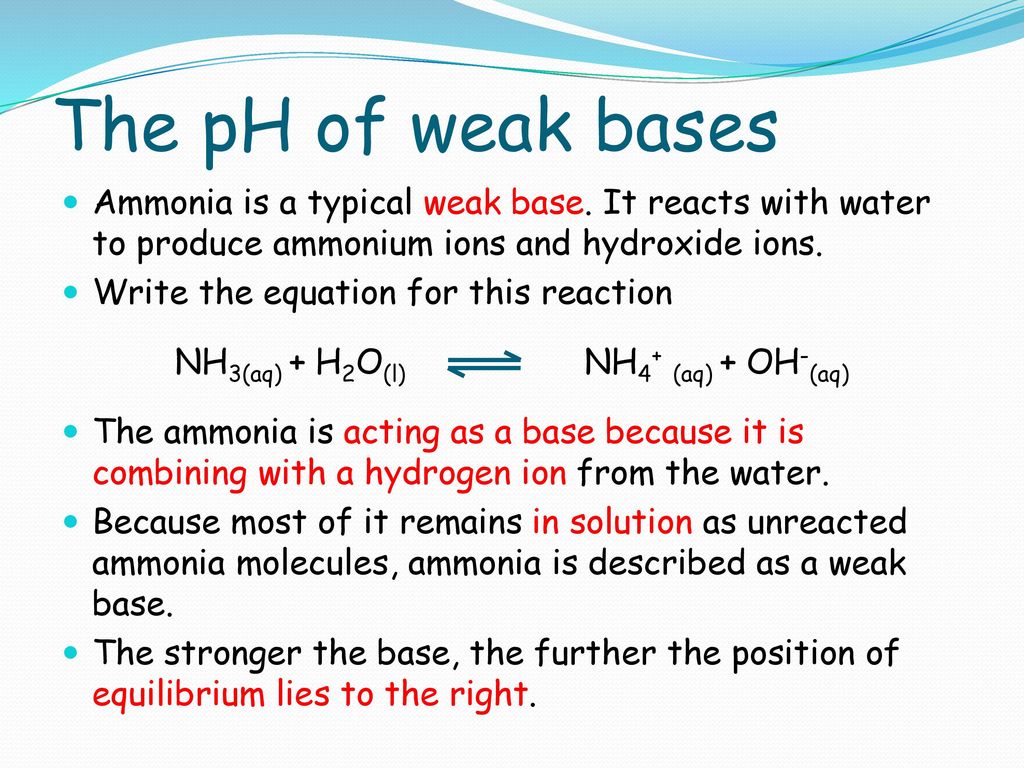
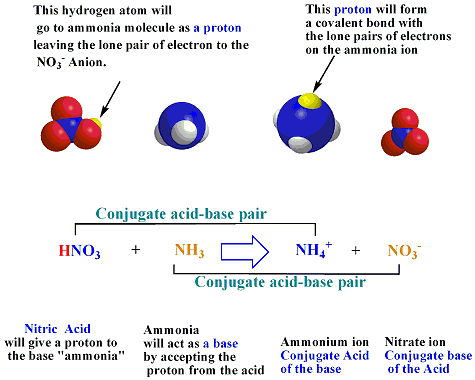
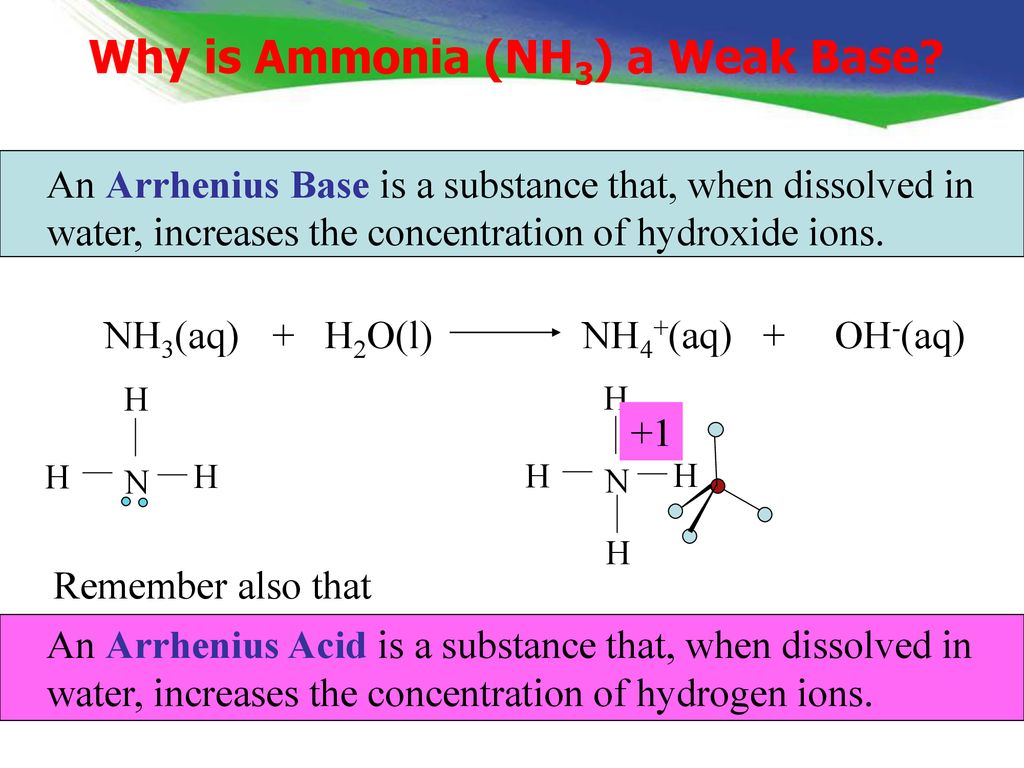
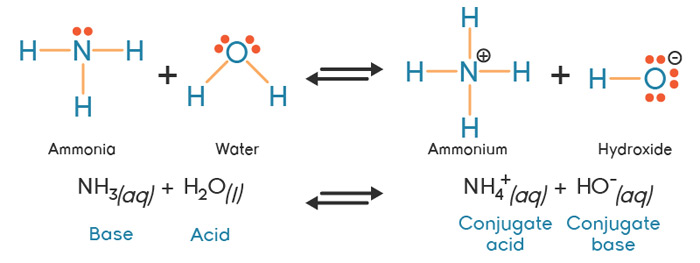
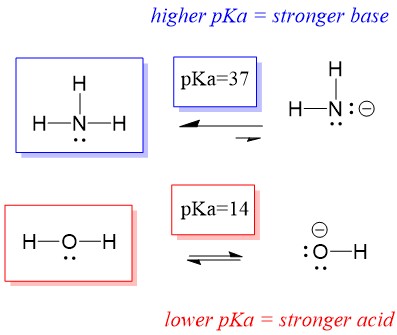
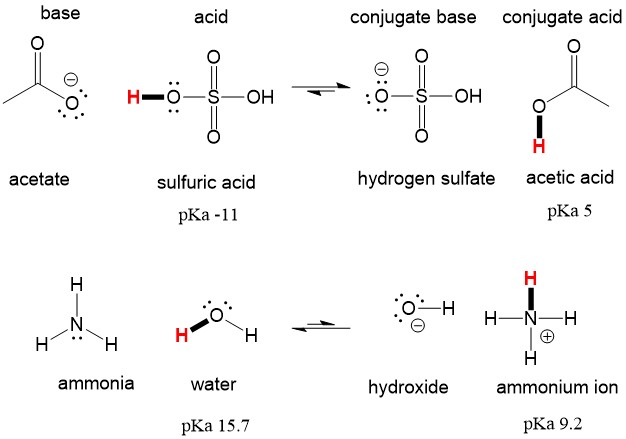
Explain the differences between the Bronsted-Lowry and the Lewis acid-base theories, using the formation of the ammonium ion from ammonia and water to illustrate your points. | Homework.Study.com
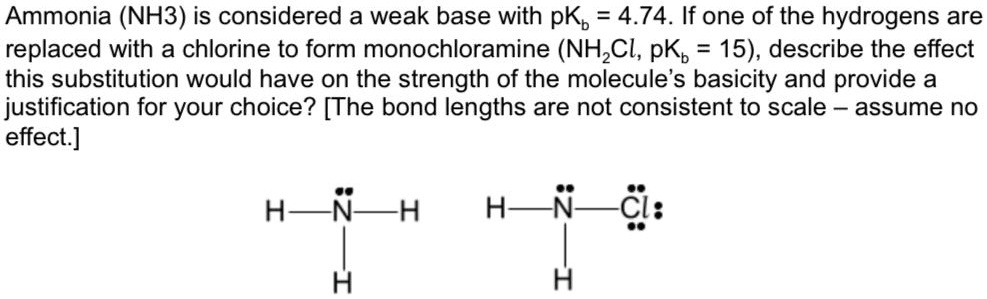
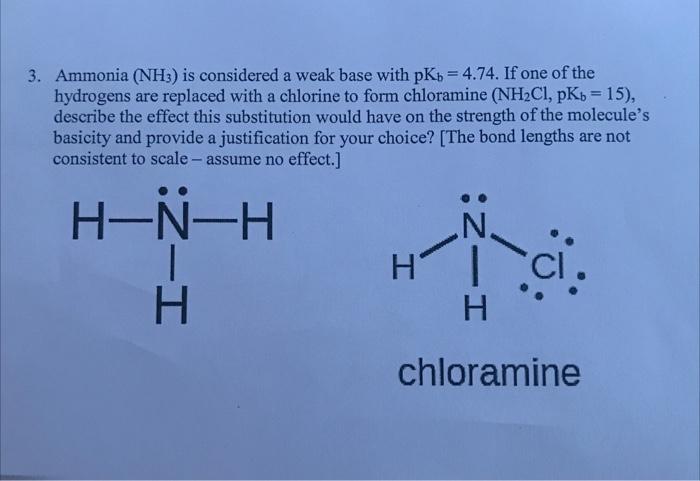
SOLVED: Ammonia (NH3) is considered a weak base with pKb = 4.74. If one of the hydrogens are replaced with a chlorine to form monochloramine (NHZCl, pKb 15) , describe the effect

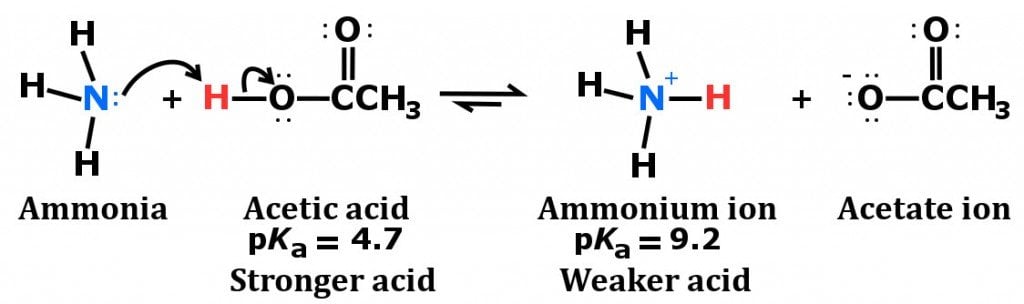
redox - Why does ammonia act as an acid in only the first of these two reactions? - Chemistry Stack Exchange